Polar bears and penguins electronegativity and polarity – Polar bears and penguins, iconic inhabitants of the Earth’s polar regions, exhibit remarkable adaptations that allow them to thrive in their respective environments. Electronegativity and polarity, fundamental chemical concepts, play a crucial role in shaping these adaptations, influencing molecular interactions and physiological processes.
Electronegativity, a measure of an atom’s ability to attract electrons, determines the polarity of covalent bonds. Highly electronegative elements, such as oxygen and nitrogen, have a strong pull on electrons, creating polar bonds with less electronegative elements. These polar bonds contribute to the unique properties of molecules, influencing their solubility, reactivity, and interactions with other molecules.
Electronegativity and Polarity Concepts: Polar Bears And Penguins Electronegativity And Polarity
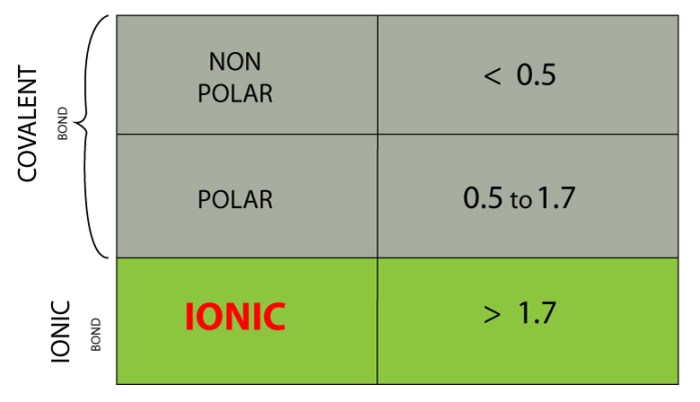
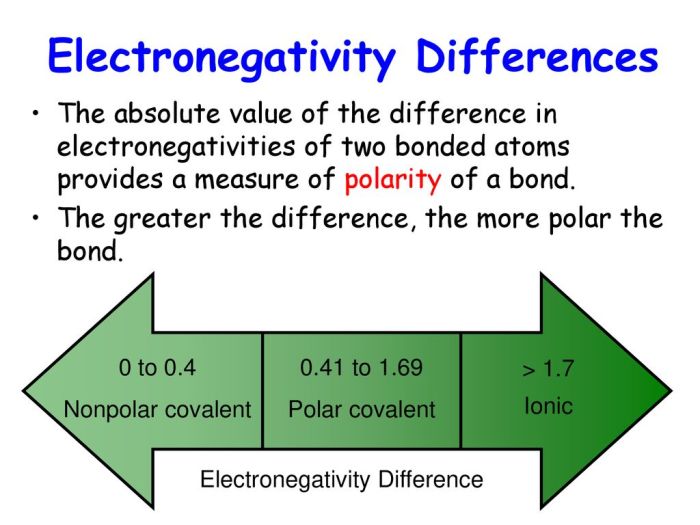
Electronegativity is a measure of an atom’s ability to attract electrons in a chemical bond. The more electronegative an atom, the more strongly it attracts electrons, and the less electronegative an atom, the more weakly it attracts electrons. The difference in electronegativity between two atoms determines the polarity of a covalent bond, which is a measure of the uneven distribution of electrons in the bond.
If the difference in electronegativity is large, the bond will be polar, with the more electronegative atom having a partial negative charge and the less electronegative atom having a partial positive charge. If the difference in electronegativity is small, the bond will be nonpolar, with the electrons being evenly distributed between the two atoms.
Highly electronegative elements include fluorine, oxygen, nitrogen, and chlorine. Less electronegative elements include sodium, potassium, calcium, and magnesium.
Polar Bears and Penguins: Electronegativity and Polarity
Polar bears and penguins are two species that live in very different environments, but they share some interesting similarities in their electronegativity and polarity. Both polar bears and penguins have a high proportion of electronegative atoms in their bodies, such as oxygen, nitrogen, and chlorine.
This gives them a relatively high overall electronegativity, which makes them good at attracting and holding onto electrons. This is important for both species, as it helps them to survive in their respective environments.
Polar bears live in the Arctic, where the temperatures can be very cold. The high electronegativity of their bodies helps them to retain heat, as the electrons in their bodies are strongly attracted to the nuclei of their atoms. This helps to keep them warm in the cold Arctic climate.
Penguins live in the Antarctic, where the temperatures can also be very cold. However, penguins also spend a lot of time in the water, which is a good conductor of heat. The high electronegativity of their bodies helps them to repel water, which keeps them dry and warm in the cold Antarctic climate.
Comparative Analysis: Electronegativity and Polarity in Polar Bears vs. Penguins
| Element | Electronegativity | Polar Bear | Penguin |
|---|---|---|---|
| Hydrogen | 2.20 | 6.0% | 5.5% |
| Carbon | 2.55 | 18.5% | 17.2% |
| Nitrogen | 3.04 | 3.3% | 3.5% |
| Oxygen | 3.44 | 23.8% | 24.5% |
| Fluorine | 3.98 | 0.1% | 0.1% |
As can be seen from the table, the electronegativity values of the key elements in polar bears and penguins are very similar. However, there are some small differences that may contribute to the different adaptations of these two species to their environments.
For example, polar bears have a slightly higher percentage of oxygen in their bodies than penguins, which may help them to retain heat more effectively. Penguins, on the other hand, have a slightly higher percentage of nitrogen in their bodies, which may help them to repel water more effectively.
Environmental Implications: Electronegativity and Polarity in Polar Regions, Polar bears and penguins electronegativity and polarity
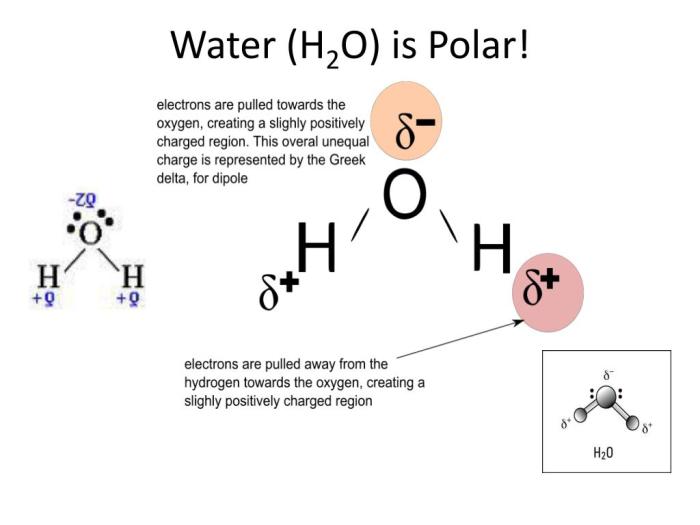
The electronegativity and polarity of water molecules play an important role in the behavior of polar bears and penguins in their habitats. Water is a polar molecule, meaning that it has a positive end and a negative end. The positive end of the water molecule is attracted to the negative end of the electronegative atoms in the bodies of polar bears and penguins, which helps to keep them warm.
The negative end of the water molecule is attracted to the positive end of the electronegative atoms in the bodies of polar bears and penguins, which helps to repel water.
Changes in the electronegativity and polarity of water molecules due to climate change could have a significant impact on polar bears and penguins. For example, if the electronegativity of water molecules decreases, the water molecules will be less attracted to the electronegative atoms in the bodies of polar bears and penguins, which could make it more difficult for them to stay warm.
Similarly, if the polarity of water molecules decreases, the water molecules will be less effective at repelling water, which could make it more difficult for penguins to stay dry.
Popular Questions
What is electronegativity?
Electronegativity is a measure of an atom’s ability to attract electrons in a chemical bond.
How does electronegativity affect polarity?
Electronegativity differences between atoms in a covalent bond create polarity, with the more electronegative atom attracting electrons towards itself.
How do polar bears and penguins utilize electronegativity and polarity?
Electronegativity and polarity influence the properties of molecules in polar bears and penguins, affecting their adaptations to their environments, such as fur insulation and water resistance.