Embark on an illuminating journey with the Bohr model introduction gizmo answer key, a comprehensive guide that unveils the intricacies of atomic structure. Delve into the historical context, fundamental principles, and limitations of this pioneering model, gaining a deeper understanding of the foundation of modern atomic theory.
The Bohr model, a groundbreaking concept proposed by Niels Bohr in 1913, revolutionized our comprehension of atomic structure. This model introduced the concept of quantized energy levels, providing a framework for understanding the behavior of electrons within atoms. The Bohr model laid the groundwork for subsequent advancements in quantum mechanics, shaping our understanding of the fundamental building blocks of matter.
Bohr Model Introduction: Bohr Model Introduction Gizmo Answer Key
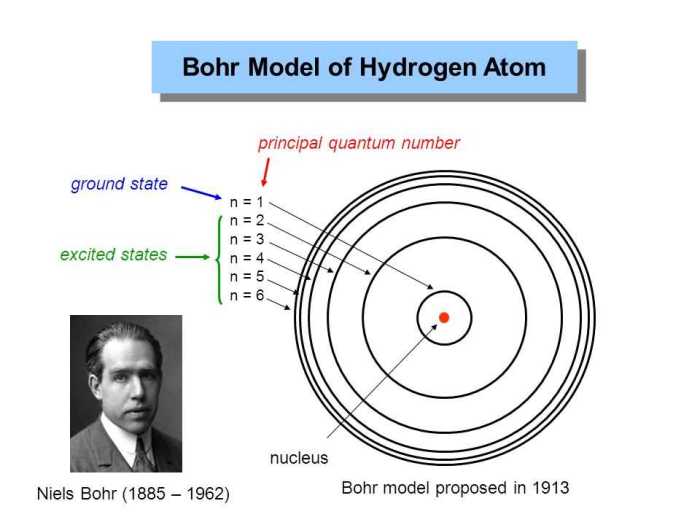
The Bohr model, proposed by Niels Bohr in 1913, was a groundbreaking theory that revolutionized our understanding of atomic structure. It marked a significant departure from the classical model and paved the way for modern quantum mechanics.
Key Features of the Bohr Model
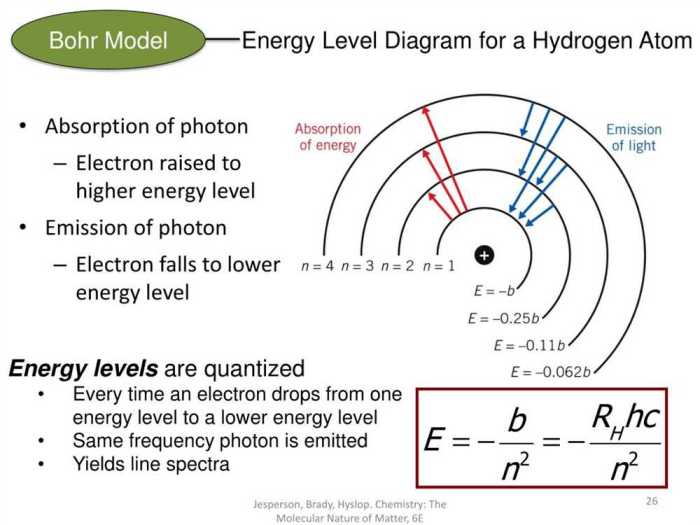
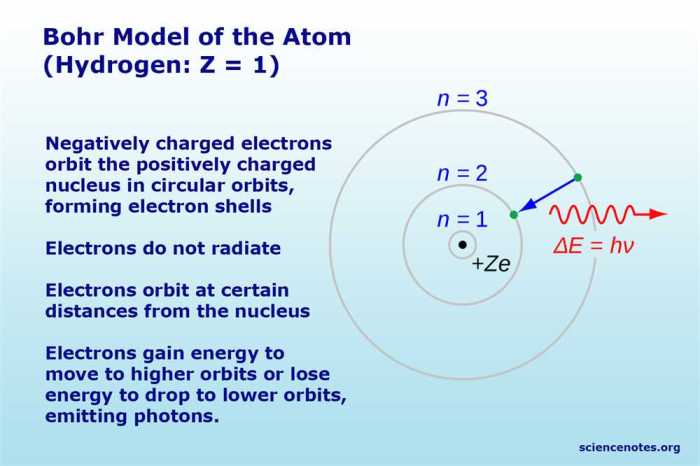
- Electrons orbit the nucleus in discrete, circular paths called energy levels.
- Each energy level has a specific energy value.
- Electrons can only exist in specific energy levels and can transition between them by absorbing or emitting photons.
- The energy of a photon is equal to the difference in energy between the two energy levels involved in the transition.
Energy Levels and Transitions
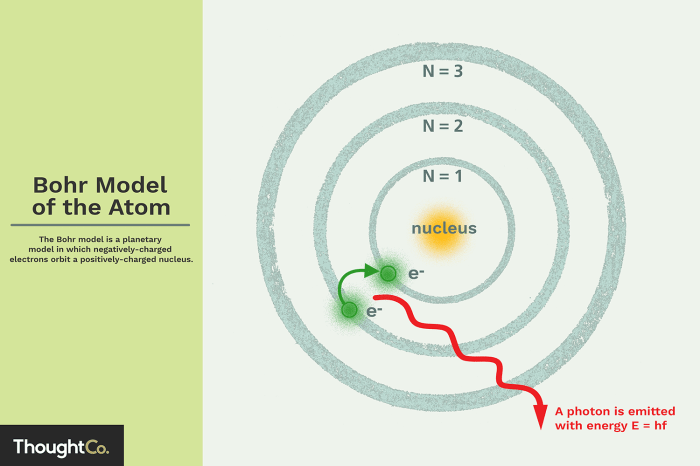
According to the Bohr model, electrons occupy energy levels that are quantized, meaning they can only exist in certain discrete values. The energy of an electron is inversely proportional to its distance from the nucleus. The lowest energy level, known as the ground state, is closest to the nucleus, followed by higher energy levels.
Electrons can transition between energy levels by absorbing or emitting photons. When an electron absorbs a photon, it moves to a higher energy level. Conversely, when an electron emits a photon, it transitions to a lower energy level.
Limitations of the Bohr Model
While the Bohr model was a significant step forward in our understanding of atomic structure, it had several limitations:
- It could not explain the spectra of more complex atoms.
- It did not account for the wave-particle duality of electrons.
- It could not explain the fine structure of spectral lines.
Applications of the Bohr Model
Despite its limitations, the Bohr model has several applications:
- It provides a simple and intuitive picture of atomic structure.
- It can be used to calculate the energy levels of simple atoms.
- It has been used to develop more sophisticated models of atomic structure.
Gizmo Answer Key, Bohr model introduction gizmo answer key
| Question | Answer | Explanation |
|---|---|---|
| What is the energy of an electron in the ground state of hydrogen? | -13.6 eV | The energy of an electron in the ground state of any atom is equal to
|
| What is the wavelength of a photon emitted when an electron transitions from the n = 3 to the n = 2 energy level in hydrogen? | 656 nm | The wavelength of a photon is given by the equation λ = hc/E, where h is Planck’s constant, c is the speed of light, and E is the energy of the photon. |
| What is the frequency of a photon absorbed when an electron transitions from the n = 2 to the n = 3 energy level in hydrogen? | 4.57 x 10^14 Hz | The frequency of a photon is given by the equation f = c/λ, where c is the speed of light and λ is the wavelength of the photon. |
Questions and Answers
What is the Bohr model?
The Bohr model is a simplified representation of an atom, proposed by Niels Bohr in 1913. It depicts electrons orbiting the nucleus in discrete, quantized energy levels.
How does the Bohr model explain electron transitions?
According to the Bohr model, electrons can transition between energy levels by absorbing or emitting photons. The energy of the photon corresponds to the difference in energy between the two levels.
What are the limitations of the Bohr model?
The Bohr model does not account for the wave-particle duality of electrons, the uncertainty principle, or the interactions between multiple electrons within an atom.